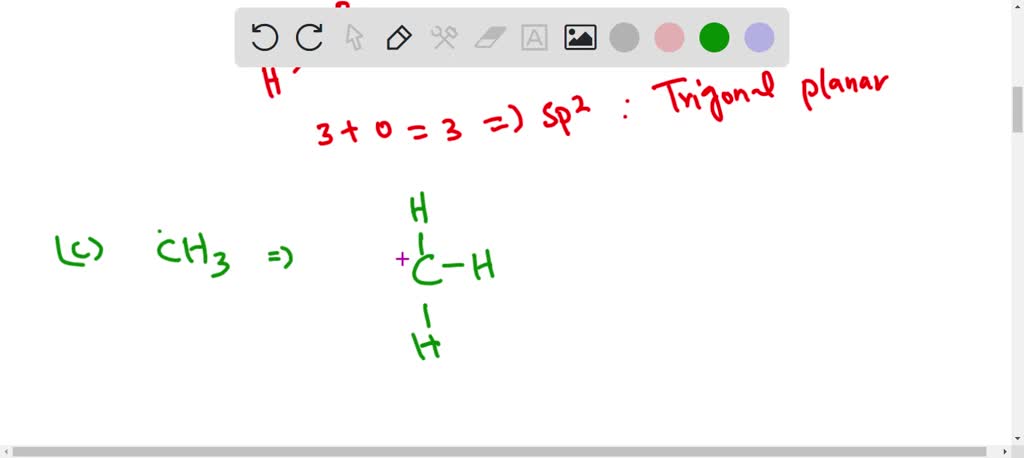
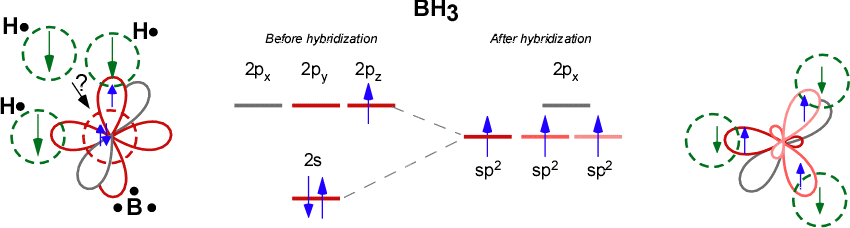
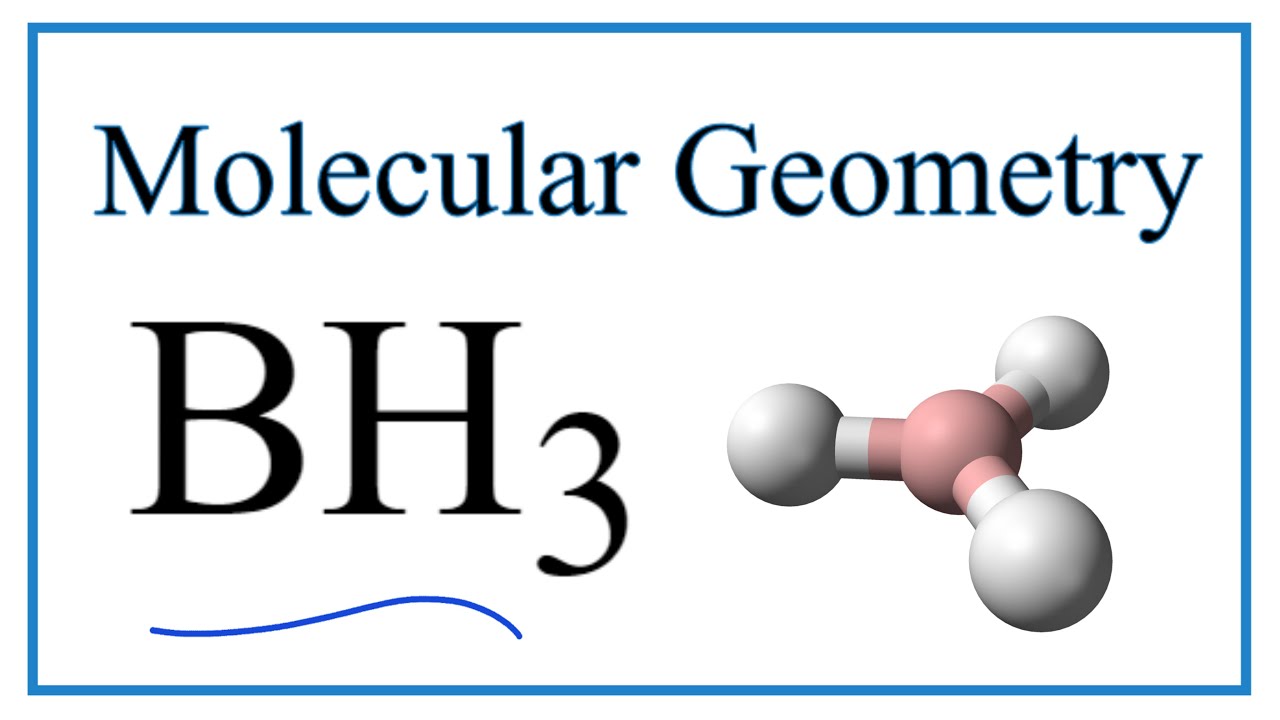
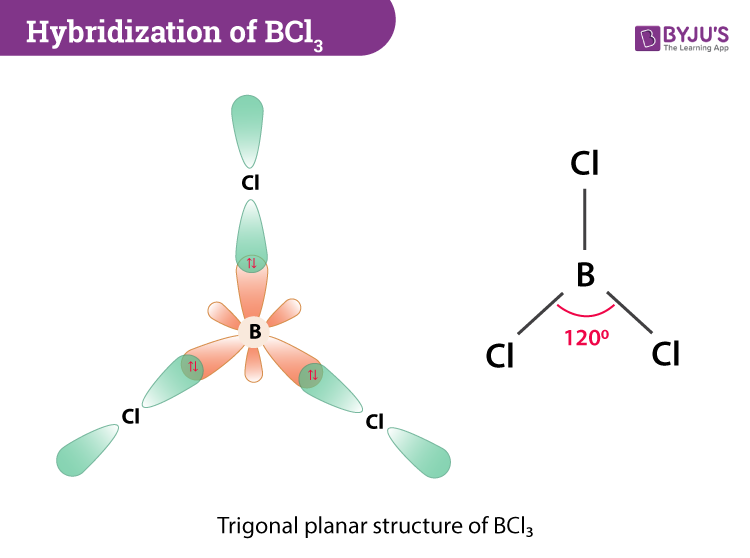
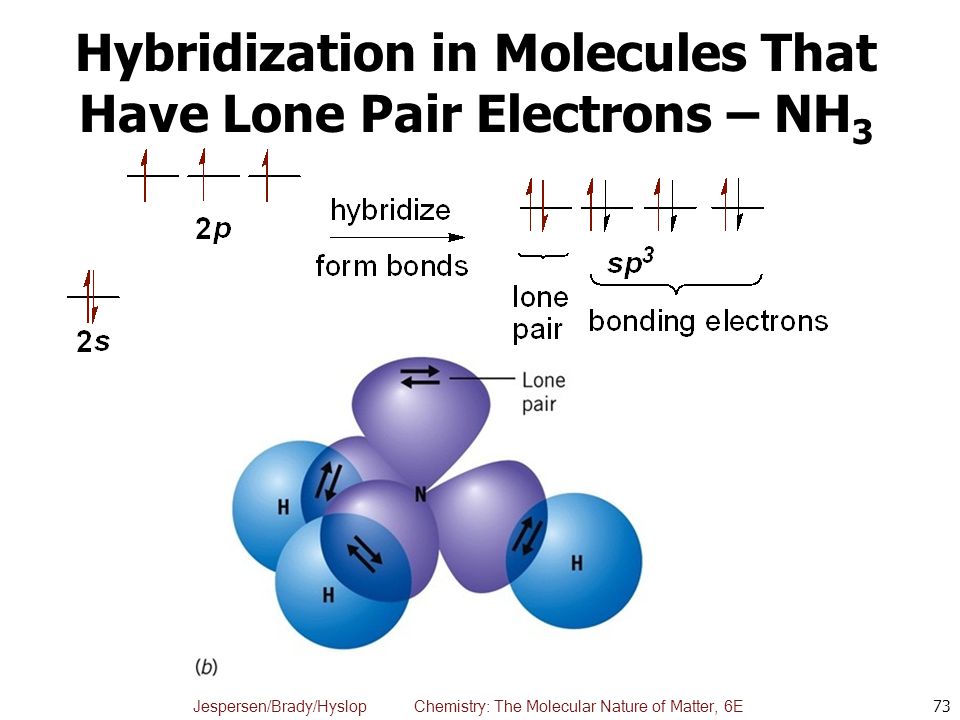
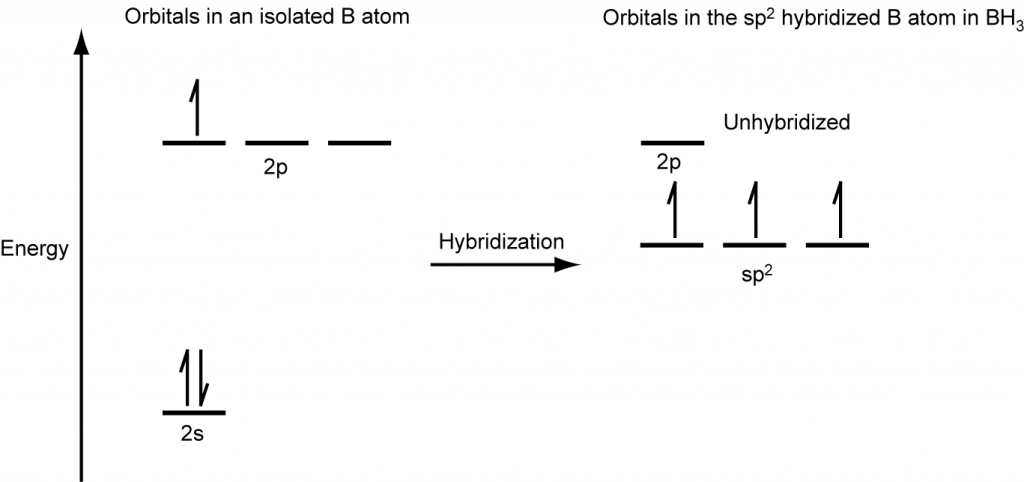
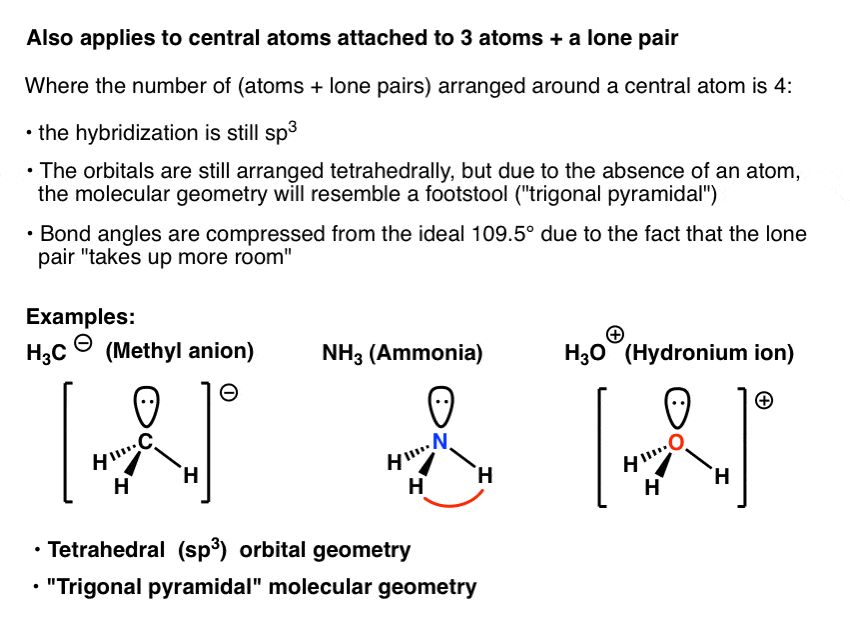
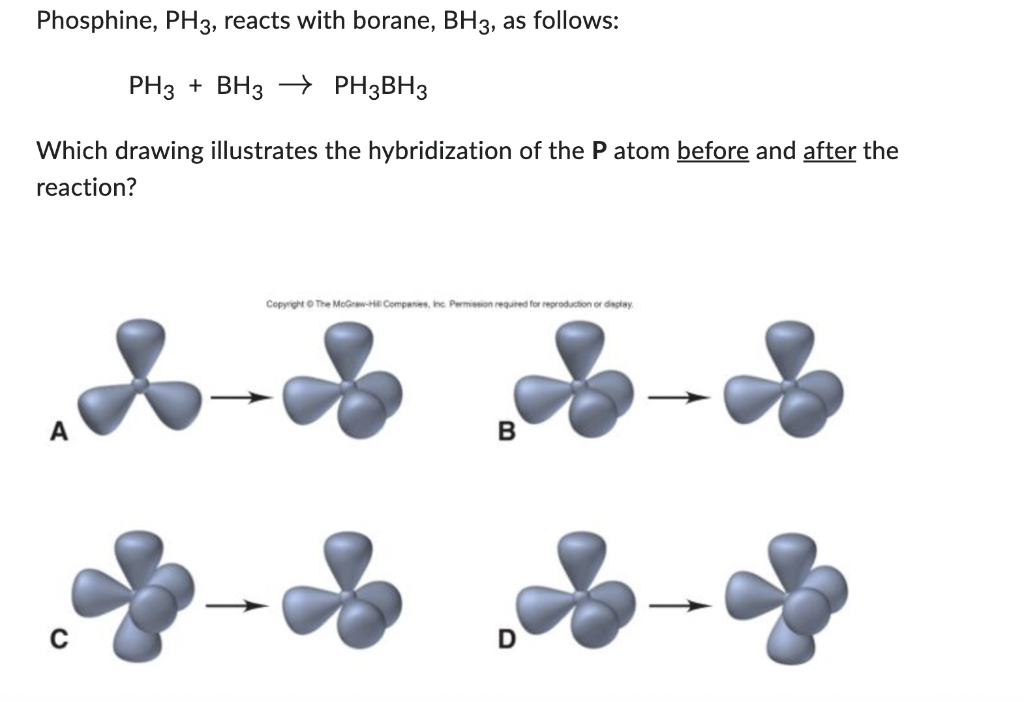
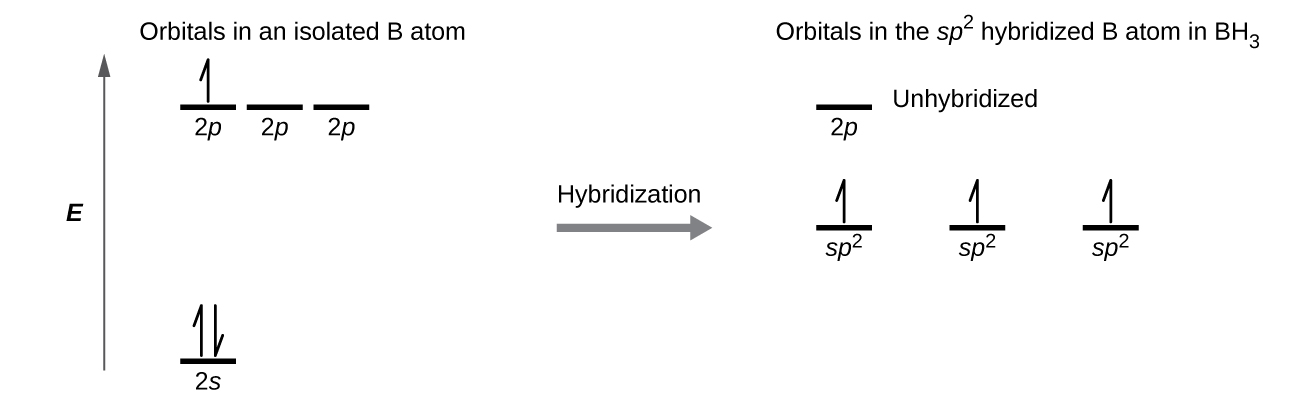
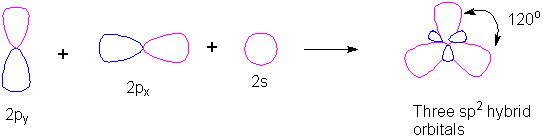Discuss the orbital structures of the following molecules on the basis of hybridisation, (i) BH3 (ii) C2H2 - Science City - Quora

The B-H bond distances are about the same in BH3 and BH4-. however, the B-F bond distance in BF3 is shorter than that in the BF4- ion. Explain. | Homework.Study.com
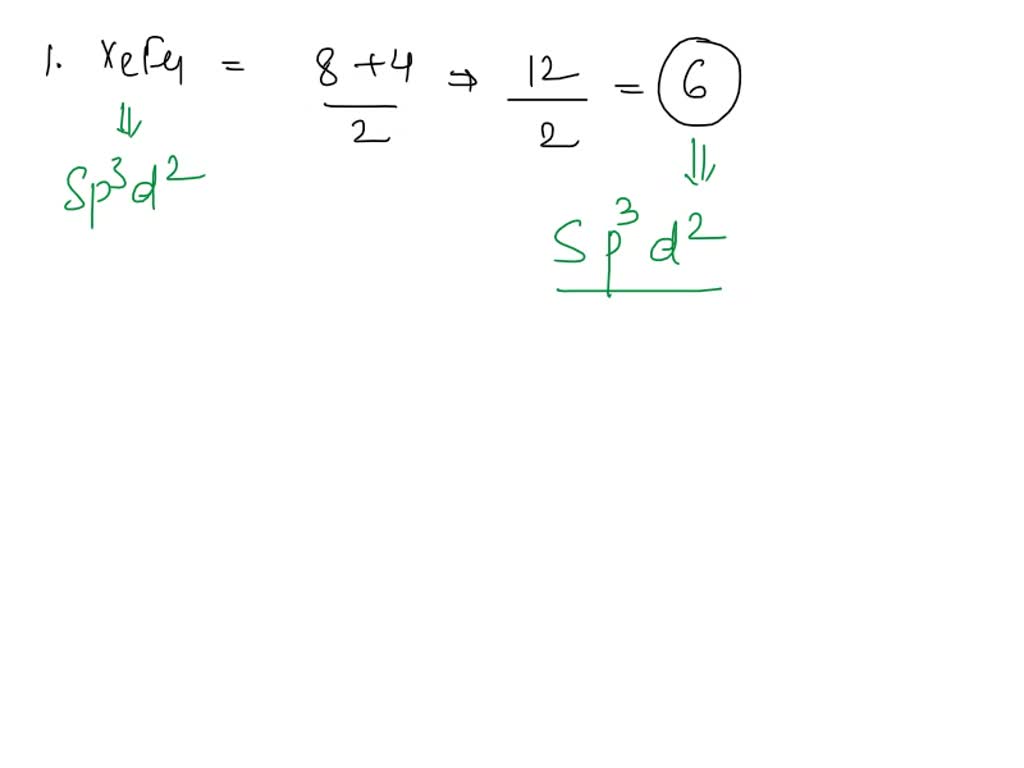
SOLVED: Give the expected hybridization of the central atom for the following molecules or ions: 1. XeF4 2. PH3 3. SO4^2- 4. BH3 5. PO3^3- 6. ClO2- 7. SO4^2-

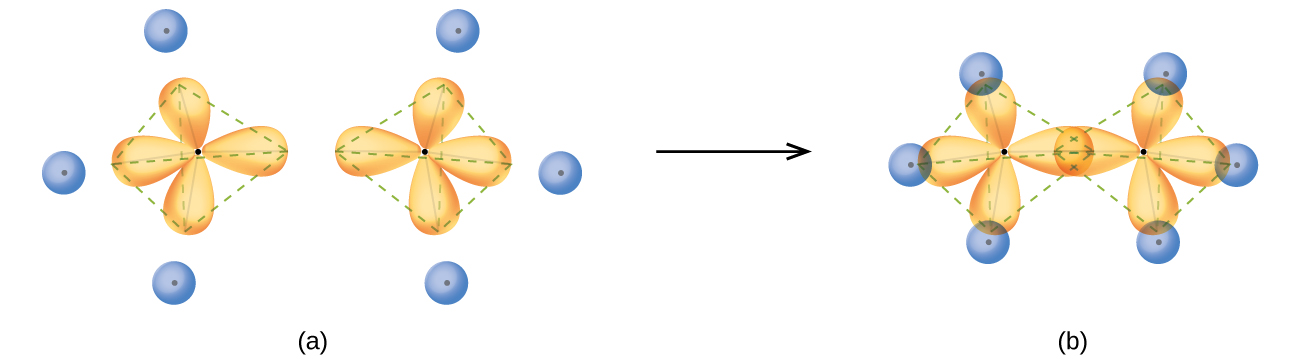
The state of hybridisation of central atom in dimer of B{H}_{3} and Be{H}_{2}?s{ p }^{ 3 },s{ p }^{ 3 }s{ p }^{ 3 },s{ p }^{ 2 }s{ p }^{ 2 },
![The state of hybridization of the central atom in dimer of \\[B{H_3}\\] and \\[Be{H_2}\\] ?A. \\[s{p^2},s{p^2}\\]B. \\[s{p^3},s{p^2}\\]C. \\[s{p^3},s{p^3}\\]D. \\[s{p^2},sp\\] The state of hybridization of the central atom in dimer of \\[B{H_3}\\] and \\[Be{H_2}\\] ?A. \\[s{p^2},s{p^2}\\]B. \\[s{p^3},s{p^2}\\]C. \\[s{p^3},s{p^3}\\]D. \\[s{p^2},sp\\]](https://www.vedantu.com/question-sets/258693db-7817-497f-a15b-137735afe7095512141555770833478.png)
The state of hybridization of the central atom in dimer of \\[B{H_3}\\] and \\[Be{H_2}\\] ?A. \\[s{p^2},s{p^2}\\]B. \\[s{p^3},s{p^2}\\]C. \\[s{p^3},s{p^3}\\]D. \\[s{p^2},sp\\]