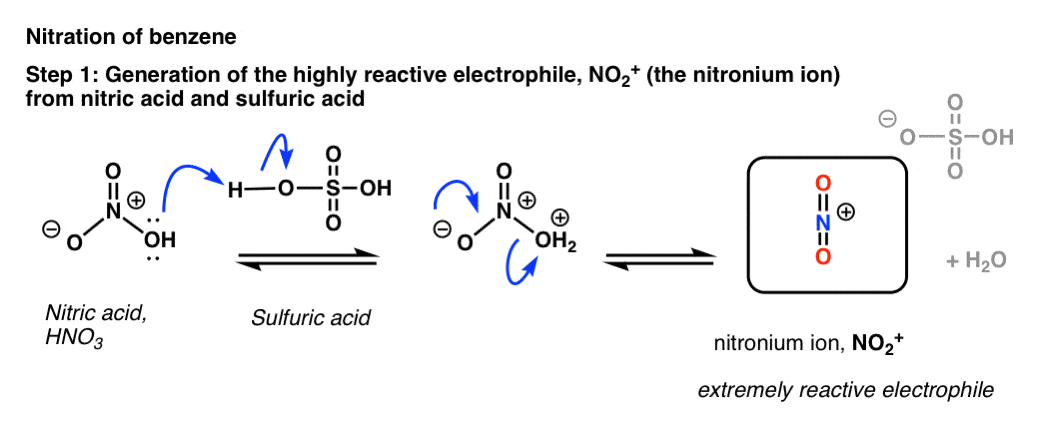
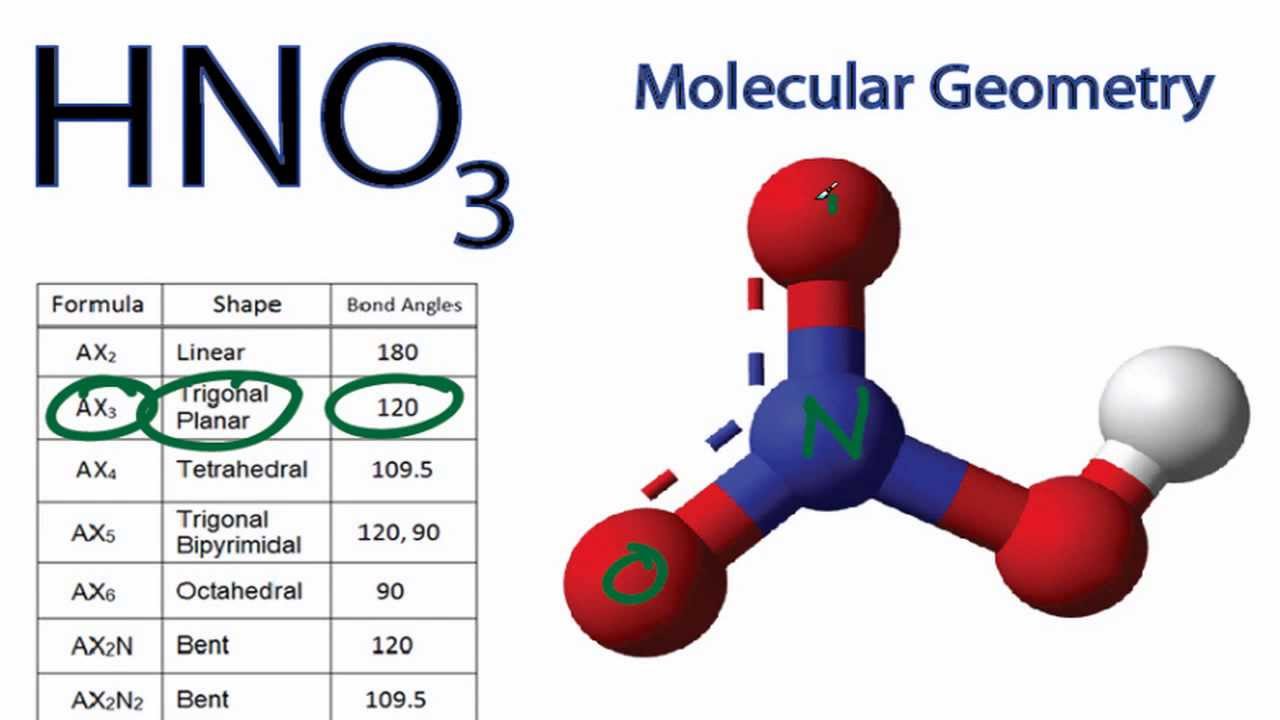
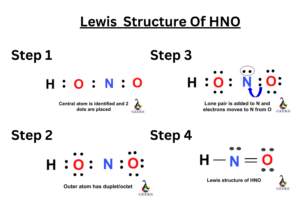
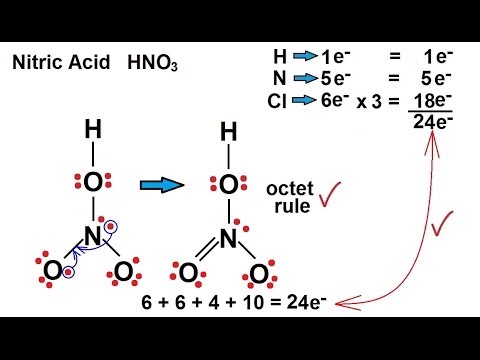
Nitric acid (HNO3) Lewis structure, molecular geometry, hybridization, polar or nonpolar | Molecular geometry, Molecular, Electron configuration

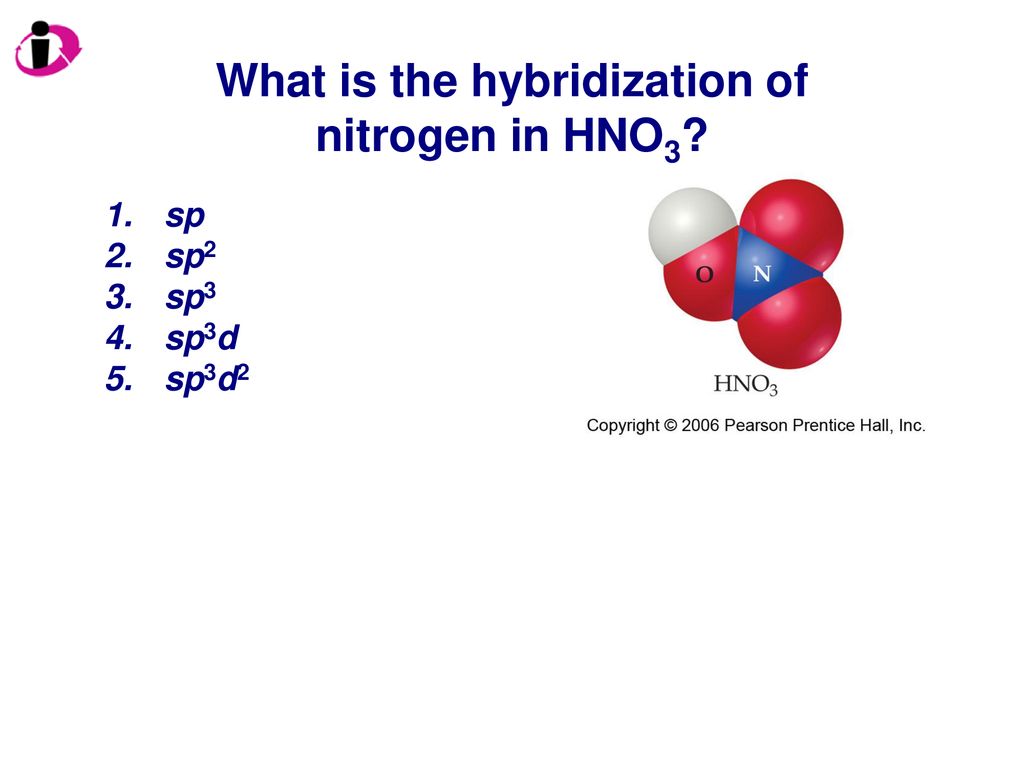
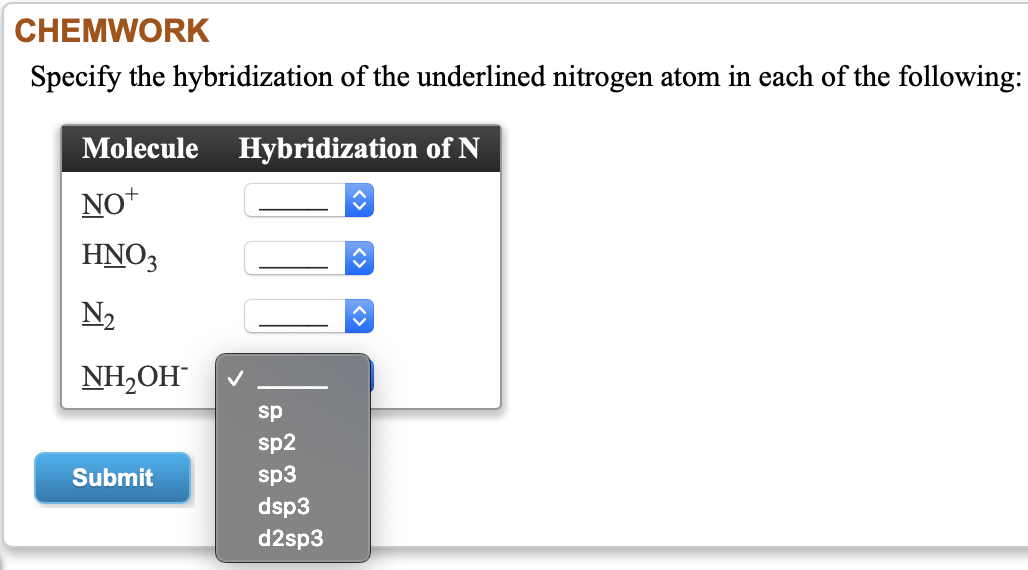
Provide the following information for the compound HNO3. a. Lewis dot structure b. hybridization c. electron geometry d. molecular geometry e. polarity | Homework.Study.com
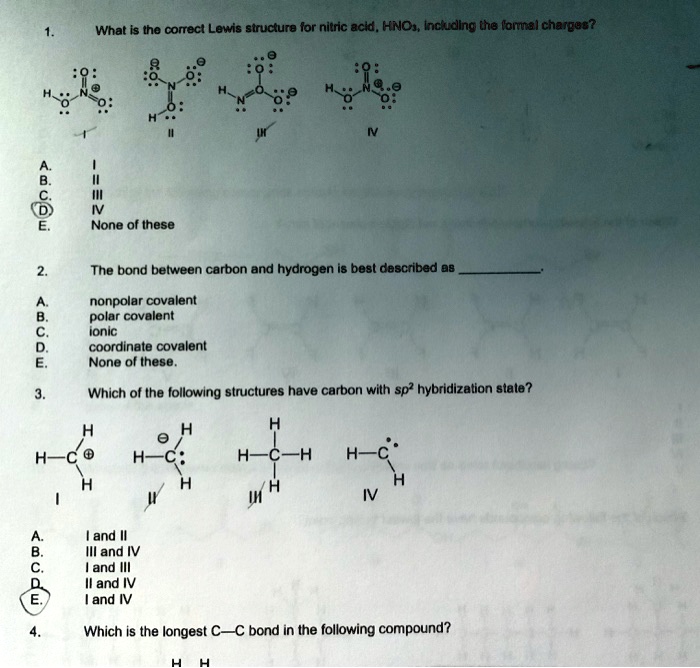
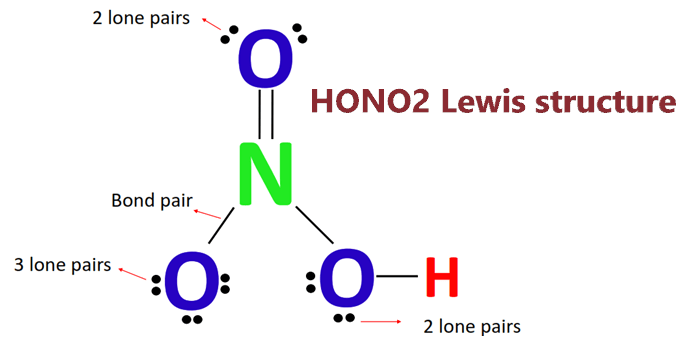
SOLVED: What is the correct Lewis structure for nitric acid, HNO3, including the formal charges? None of these. The bond between carbon and hydrogen is best described as nonpolar covalent. Which of
In HNO3 why does the nitrogen atom give electrons to the third oxygen when it already satisfied its valency with two oxygens one with a single bond and one with a double
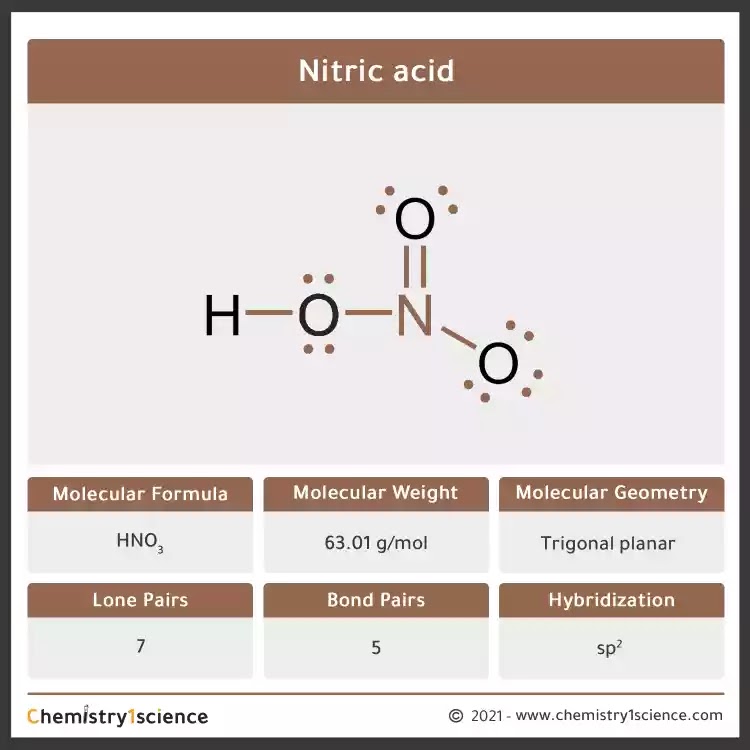
Nitric acid: Molecular Geometry - Hybridization - Molecular Weight - Molecular Formula - Bond Pairs - Lone Pairs - Lewis structure – infographic